Filter the 100ml of drinking water sample and transfer the filter paper quite diligently in 100ml Soybean Casein
VALIDATION OF NEUTRALIZATION METHODS—RECOVERY COMPARISONS A validated method for neutralizing the antimicrobial Attributes of an item should meet up with two standards: neutralizer efficacy and neutralizer toxicity. The validation examine paperwork the neutralization method used is efficient in inhibiting the antimicrobial Attributes on the products (neutralizer efficacy) devoid of impairing the Restoration of viable microorganisms (neutralizer toxicity). Validation protocols could meet these two standards by evaluating Restoration outcomes for therapy teams.
The volume of contaminated practical bacteria (yeasts and mildew) from the unit mass, quantity, or space (g, ml, or 10cm2) in the drug is detected, the result of which may be applied to guage the diploma of contamination of your medicine and to evaluate the sanitary top quality with the drugs
Evaluating with constructive and adverse controls, the absence of the Coagulase response indicates the absence of Staphylococcus aureus
Microbial limit test is carried out to ascertain no matter if drug products adjust to an established specification for microbial top quality. Creator Identify: Helen
Note that fewer pure plant steam may very well be utilized for steam sterilization of nonporous loads, common cleansing and sterilization of nonproduct contact devices and analytical resources, humidification of air in nonmanufacturing places, where applied like a nonproduct Get hold of warmth exchange medium, and in all appropriate apps associated with bulk pharmaceutical chemical and API manufacture.
The next all interact to generate some unusual and surprising retention phenomena for h2o procedure microorganisms: the variability within the assortment and regular pore measurements designed by the different membrane fabrication procedures, the variability of your area chemistry and 3-dimensional framework relevant to the several polymers Utilized in these filter matrices, and the scale and area Attributes of your microorganism meant to be retained because of the filters. B. diminuta may well not the ideal obstacle microorganisms for demonstrating bacterial retention for 0.two- to 0.22-µm rated filters to be used in drinking water programs since it appears to become more very easily retained by these filters than some water method flora.
This issue is mentioned in detail down below. The second thing to consider is the incubation problems. Optimum disorders for expansion have to be existing to guarantee total advancement and reproducible benefits.
Biochemical test or identification by automated methods can be used for confirmatory identification.
If ANOVA is used, and important dissimilarities Amongst the populations are decided, a test which include Dunnett's test could be applied, with the peptone group utilized given that the control group.
These aspects also have an effect on the validation of Restoration methods for aqueous or nonaqueous products, regardless of their antimicrobial Qualities; thus, all test methods need to be validated with these elements in your mind.
Due safeguards ought to be taken to stay more info away from contamination have to be this sort of that they don't influence any microorganisms which have been to get exposed during the test.
The character on the challenge microorganism exerts a strong impact on the reaction for the antimicrobial agent, and so on the neutralization necessary for recovery.
A validation approach for just a drinking water method ordinarily features the next measures: (one) developing requirements for high-quality characteristics with the finished h2o along with the resource drinking water; (two) defining ideal device operations as well as their working parameters for acquiring the specified completed water good quality characteristics through the available supply h2o; (3) deciding upon piping, tools, controls, and checking technologies; (four) producing an IQ stage consisting of instrument calibrations, inspections to confirm which the drawings accurately depict the final configuration with the water technique and, where needed, Exclusive tests to verify the set up satisfies the design specifications; (five) acquiring an OQ stage consisting of tests and inspections to verify the machines, method alerts, and controls are operating reliably and click here that ideal warn and action amounts are recognized (This section of qualification might overlap with facets of the subsequent move.
 Joseph Mazzello Then & Now!
Joseph Mazzello Then & Now! Shane West Then & Now!
Shane West Then & Now!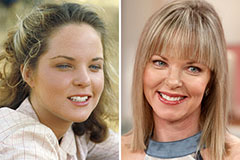 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Mary Beth McDonough Then & Now!
Mary Beth McDonough Then & Now! Christy Canyon Then & Now!
Christy Canyon Then & Now!